
Главная страница Случайная страница
КАТЕГОРИИ:
АвтомобилиАстрономияБиологияГеографияДом и садДругие языкиДругоеИнформатикаИсторияКультураЛитератураЛогикаМатематикаМедицинаМеталлургияМеханикаОбразованиеОхрана трудаПедагогикаПолитикаПравоПсихологияРелигияРиторикаСоциологияСпортСтроительствоТехнологияТуризмФизикаФилософияФинансыХимияЧерчениеЭкологияЭкономикаЭлектроника
Classification of organic compounds
|
|
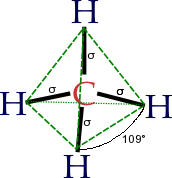 The concept of functional groups is central in organic chemistry, both as a means to classify structures and for predicting properties. A functional group is a molecular module, and the reactivity of that functional group is assumed, within limits, to be the same in a variety of molecules. Functional groups can have decisive influence on the chemical and physical properties of organic compounds. Molecules are classified on the basis of their functional groups. Alcohols, for example, all have the subunit C-O-H. All alcohols tend to be somewhat hydrophilic, usually form esters, and usually can be converted to the corresponding halides. Most functional groups feature heteroatoms (atoms other than C and H). Organic compounds are classified according to functional groups: alcohols, carboxylic acids, amines, etc.
The concept of functional groups is central in organic chemistry, both as a means to classify structures and for predicting properties. A functional group is a molecular module, and the reactivity of that functional group is assumed, within limits, to be the same in a variety of molecules. Functional groups can have decisive influence on the chemical and physical properties of organic compounds. Molecules are classified on the basis of their functional groups. Alcohols, for example, all have the subunit C-O-H. All alcohols tend to be somewhat hydrophilic, usually form esters, and usually can be converted to the corresponding halides. Most functional groups feature heteroatoms (atoms other than C and H). Organic compounds are classified according to functional groups: alcohols, carboxylic acids, amines, etc.
Substances consisting entirely of single-bonded carbon and hydrogen atoms and lacking functional groups are called alkanes. There are three basic types of structure that they are classified in: the linear straight-chain alkanes, branched alkanes, and also cycloalkanes. Alkanes can be subdivided into three groups. An alkane is also called saturated hydrocarbon because the compound has only single bonds between the atoms. Alkanes that have a ring or cyclic structure are called cyclic alkanes, while those that are without are called acyclic. General formula: CnH2n+2, n> 2.
Alkenes area class of hydrocarbons that contain only carbon and hydrogens. They are unsaturated compounds that contain at least one carbon-to-carbon double bond. Another term that is often used to describe alkenes is olefins.
Alkynes are organic molecules made of the functional group carbon-carbon triple bonds. They are written in the empirical formula of CnH2n-2. They are unsaturated hydrocarbons.
Benzene is one of the best-known aromatic compounds as it is one of the simplest and most stable aromatics.
Aromatic hydrocarbons contain conjugated double bonds. This means that every carbon atom in the ring is sp2 hybridized, allowing for added stability. The most important example is benzene, the structure of which was formulated by Kekulé who first proposed the delocalization or resonance principle for explaining its structure.
Alcohols are molecules containing the hydroxyl functional group (-OH) that is bonded to carbon atom of an alkyl or substituted alkyl. The hydroxyl functional group strongly contributes to the physical properties of alcohols.
Compounds in which a hydroxyl group is bonded to an aromatic ring are called phenols. The chemical behavior of phenols is different in some respects from that of the alcohols, so it is sensible to treat them as a similar but characteristically distinct group.
Ethers are a class of organic compounds that contain an oxygen between two alkyl groups. They have the formula R-O-R', with R's being the alkyl groups. these compounds are used in dye, perfumes, oils, waxes and industrial use. Ethers are treated as alkanes and are named as alkoxyalkanes.
Amines are derivatives of ammonia in which one or more of the hydrogens has been replaced by an alkyl or aryl group.
Aldehydes and ketones are organic compounds which contain a carbonyl group - a carbon-oxygen double bond.
The carboxyl functional group that characterizes the carboxylic acids is unusual in that it is composed of two functional groups: (1) the carboxyl group and (2) of a hydroxyl group bonded to a carbonyl group. It is often written in condensed form as –CO2H or –COOH.
Esters are known for their distinctive odors and are commonly used for food aroma and fragrances. The general formula of an ester is RCOOR'.
Amino acids are biologically important organic compounds composed of amine (-NH2) and carboxylic acid (-COOH) functional groups, along with a side-chain specific to each amino acid. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen, though other elements are found in the side-chains of certain amino acids.
Proteins are large biological molecules, or macromolecules, consisting of one or more chains of amino acid residues. Proteins perform a vast array of functions within living organisms, including catalyzing metabolic reactions, replicating DNA, responding to stimuli, and transporting molecules from one location to another.
 Nucleic acids are polymeric macromolecules, or large biological molecules, essential for all known forms of life. Nucleic acids, which include DNA (deoxyribonucleic acid) and RNA (ribonucleic acid), are made from monomers known as nucleotides. Each nucleotide has three components: a 5-carbon sugar, a phosphate group, and a nitrogenous base. If the sugar is deoxyribose, the polymer is DNA. If the sugar is ribose, the polymer is RNA.
Nucleic acids are polymeric macromolecules, or large biological molecules, essential for all known forms of life. Nucleic acids, which include DNA (deoxyribonucleic acid) and RNA (ribonucleic acid), are made from monomers known as nucleotides. Each nucleotide has three components: a 5-carbon sugar, a phosphate group, and a nitrogenous base. If the sugar is deoxyribose, the polymer is DNA. If the sugar is ribose, the polymer is RNA.
Lipids are a large and diverse group of naturally occurring organic compounds that are related by their solubility in nonpolar organic solvents (e.g. ether, chloroform, acetone & benzene) and general insolubility in water. There is great structural variety among the lipids, as will be demonstrated in the following sections.
Carbohydrates are large biological molecules consisting of carbon, hydrogen, and oxygen atoms, usually with a hydrogen: oxygen atom ratio of 2: 1.
Polymers are long chain, giant organic molecules are assembled from many smaller molecules called monomers. Polymers consist of many repeating monomer units in long chains, sometimes with branching or cross-linking between the chains.
TASKS