Главная страница Случайная страница
КАТЕГОРИИ:
АвтомобилиАстрономияБиологияГеографияДом и садДругие языкиДругоеИнформатикаИсторияКультураЛитератураЛогикаМатематикаМедицинаМеталлургияМеханикаОбразованиеОхрана трудаПедагогикаПолитикаПравоПсихологияРелигияРиторикаСоциологияСпортСтроительствоТехнологияТуризмФизикаФилософияФинансыХимияЧерчениеЭкологияЭкономикаЭлектроника
Qualitative Synthesis. Sildenafil (mono or combination therapy) versus placebo
|
|
Sildenafil (mono or combination therapy) versus placebo. In four placebo-controlled trials158, 161, 162, 169 the efficacy and safety profiles of sildenafil and placebo were not compared (see sildenafil dose/dosage one versus dose/dosage two and sildenafil mono versus sildenafil in combination sections). Thus, results provided here are based on data obtained from 62 placebo-controlled trials.78–91, 93–99, 101, 102, 104, 105, 107–111, 115, 122, 123, 125, 126, 128, 130–135, 137, 138, 142, 143, 146, 147, 149, 151, 156, 160, 164–168, 171, 175
Harms. In the majority of the placebo-controlled trials, the proportion of patients with at least one adverse event was greater either numerically or with statistical significance for participants taking sildenafil compared with those taking placebo. For example, in one trial of flexible dose (titrated to 100 or 25 mg), 51.3 percent and 32.9 percent of patients experienced one or more adverse events in sildenafil and placebo arms, respectively (p values were not reported).125 In another study, the corresponding proportions were 59.7 percent for the sildenafil treatment group versus 29.6 percent for the placebo arm, respectively (p = 0.079).126
The most commonly observed all-cause adverse events across the trials were headache, flushing, and dyspepsia. Other adverse events were myalgia, rhinitis, cardiovascular events, flu- like symptoms, nausea, respiratory events, diarrhea, vomiting, dizziness, chest pain, urinary tract infections, depression, and anxiety. Overall, these events were less frequent for participants taking placebo compared with those taking sildenafil. These effects were usually of a mild to moderate or transient nature not requiring discontinuation of the therapy.
The occurrence of specific adverse events involving visual disturbances, including blurry vision and chromatopsia, were reported in 33 trials.79–84, 86, 88, 90, 91, 94, 95, 97, 98, 107, 109, 115, 122, 125, 126, 131, 135, 137, 138, 142, 146, 147, 151, 156, 164, 165, 167 The percentage of patients experiencing visual side effects across the trials ranged from 1 percent88, 137, 165 to 57 percent164 for participants taking sildenafil, and from 0 percent80, 87, 88, 94, 95, 98, 101, 107, 109, 122, 125, 126, 135, 138, 147, 165, 167, 171 to 61.9 percent164 for participants taking placebo.
Cardiovascular events were reported in 18 studies.79, 83, 84, 87–89, 94, 96, 97, 101, 102, 107, 109, 125, 126, 137, 143, 166 These events were numerically more frequent in participants treated with sildenafil, ranging from 3 percent94 to 29 percent, 97 compared with the range of 0 percent101 to 12 percent97 for placebo-treated participants.
A few studies reported the need for dose reduction as a result of adverse events.80, 84, 87, 115, 151 The reasons for dose reduction were headache, 80, 87, 151 flushing, 87 chest tightness, 87 nasal stuffiness, 87 and visual disturbance.80
Twenty-four trials reported the absence of withdrawals due to adverse events.78–80, 82, 85, 98, 104, 105, 107, 110, 112, 128, 131, 133, 134, 138, 146, 149, 156, 167, 168, 171 The rate of WDE (presented as the proportion of patients who withdrew) in sildenafil treatment groups was under 5 percent in 18 trials, 81, 83, 84, 88, 90, 91, 96, 109, 115, 125, 135, 137, 142, 151, 156, 160, 175 and up to 8 to 12.5 percent in four trials.96, 99, 101, 126 In the majority of these trials, the rate for withdrawals due to adverse events in placebo-treated participants ranged between 2 and 8.5 percent. The specific events leading to withdrawals were headache, 88, 101, 109, 137, 142, 151 nausea, vomiting, gastrointestinal symptoms, 86, 88, 137 visual disturbances, 88, 165 cardiovascular events, 87, 89, 99, 101, 165, 166 urinary tract infection, 166 chest pain, 101 and cerebrovascular events.160 These events were reported for participants treated with sildenafil, with the exception of one case of myocardial infarction89 and one case of urinary tract infection166 in placebo-treated participants.
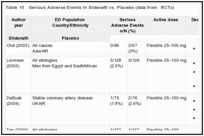
The occurrence/absence of serious adverse events was reported in 51 trials. In 29 trials, no patient experienced any serious adverse event.78, 80, 81, 85, 90, 91, 98, 99, 101, 105, 108, 110–112, 124, 125, 128, 131, 133, 134, 138, 146, 149, 156, 157, 167, 168, 173 Thus, the occurrence of serious adverse events was reported in 22 trials (Table 10).82–84, 87–90, 93, 95–97, 107, 109, 123, 126, 135, 143, 147, 151, 160, 166, 171 In general, the quality of reporting serious adverse events was poor, and some studies did not provide a full description of events.82, 95, 107, 109, 126, 135, 147, 151, 160, 166 In total, 95 participants had at least one serious adverse event while taking sildenafil or placebo, of which 32 were taking sildenafil83, 84, 87, 88, 90, 93, 97, 107, 109, 126, 135, 143, 147, 151, 160, 166 and 36 were taking placebo.82, 84, 87, 89, 97, 107, 109, 123, 126, 135, 143, 147, 151, 160, 166, 171 For the remaining 27 participants in two trials, 95, 96 the treatment group designation was not reported. Cardiovascular events were the most frequent category of serious adverse events. These included myocardial infarctions, which occurred in one participant taking sildenafil, 83 two participants taking placebo, 89, 126 and one participant whose group designation was unknown.96 Severe angina pectoris occurred in a participant taking 100 mg sildenafil87 and in another patient taking placebo.84 Heart failure, atrial fibrillation, and arrhythmia occurred in two participants taking sildenafil.143 Cerebrovascular events occurred in two participants taking sildenafil, 143, 160 one of which was taking 100 mg of sildenafil.160 Respiratory events included pneumococcal pneumonia in one participant on placebo143 and pulmonary edema in another participant on sildenafil.143 Accidental injuries were reported in two participants, one severe vertebral fracture in a participant taking sildenafil, 83 and the other a hand injury in a participant taking placebo.87