
Главная страница Случайная страница
КАТЕГОРИИ:
АвтомобилиАстрономияБиологияГеографияДом и садДругие языкиДругоеИнформатикаИсторияКультураЛитератураЛогикаМатематикаМедицинаМеталлургияМеханикаОбразованиеОхрана трудаПедагогикаПолитикаПравоПсихологияРелигияРиторикаСоциологияСпортСтроительствоТехнологияТуризмФизикаФилософияФинансыХимияЧерчениеЭкологияЭкономикаЭлектроника
Fundamental concepts of chemistry
|
|
chemical formula, chemical equation, proton, neutron, element, electron, atomic nucleus, molecule, cation, anion, chemical compound, chemical reaction, chemical bonds, ion, molecule, atomic number
An atom is a collection of matter consisting of a positively charged core (the ________________) which contains ____________ and ____________ and which maintains a number of electrons to balance the positive charge in the nucleus. The atom is also the smallest portion into which an ____________ can be divided and still retain its properties, made up of a dense, positively charged nucleus surrounded by a system of ____________.
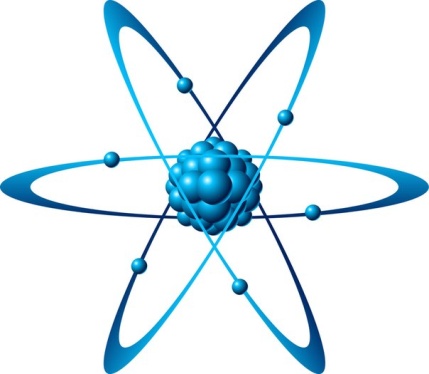 The most basic chemical substances are the chemical elements. They are building blocks of all other substances. An element is a class of atoms which have the same number of protons in the nucleus. This number is known as the ___________ ____________ of the element. For example, all atoms with 6 protons in their nuclei are atoms of the chemical element carbon, and all atoms with 92 protons in their nuclei are atoms of the element uranium. Each chemical element is made up of only one kind of atom. The atoms of one element differ from those of all other elements. Chemists use letters of the alphabet as symbols for the elements. In total, 117 elements have been observed as of 2007, of which 94 occur naturally on Earth. Others have been produced artificially.
The most basic chemical substances are the chemical elements. They are building blocks of all other substances. An element is a class of atoms which have the same number of protons in the nucleus. This number is known as the ___________ ____________ of the element. For example, all atoms with 6 protons in their nuclei are atoms of the chemical element carbon, and all atoms with 92 protons in their nuclei are atoms of the element uranium. Each chemical element is made up of only one kind of atom. The atoms of one element differ from those of all other elements. Chemists use letters of the alphabet as symbols for the elements. In total, 117 elements have been observed as of 2007, of which 94 occur naturally on Earth. Others have been produced artificially.
An ____________ is an atom or a molecule that has lost or gained one or more electrons. Positively charged ____________ (e.g. sodium cation Na+) and negatively charged ___________ (e.g. chloride Cl−) can form neutral salts (e.g. sodium chloride NaCl). Electrical forces at the atomic level create _____________ __________ that join two or more atoms together, forming ____________. Some molecules consist of atoms of a single element.
 Oxygen molecules, for example, are made up of two oxygen atoms. Chemists represent the oxygen molecule O2. The 2 indicates the number of atoms in the molecule.
Oxygen molecules, for example, are made up of two oxygen atoms. Chemists represent the oxygen molecule O2. The 2 indicates the number of atoms in the molecule.
When atoms of two or more different elements bond together, they form a ____________________. Water is a compound made up of two hydrogen atoms and one oxygen atom. The __________ _________ for a water molecule is H2O.
Compounds are formed or broken down by means of ____________ __________. All chemical reactions involve the formation or destruction of chemical bonds. Chemists use ___________ ___________ to express what occurs in chemical reactions. Chemical equations consist of chemical formulas and symbols that show the substances involved in chemical change.
For example, the equation
C + O2 = CO2
expresses the chemical change that occurs when one carbon atom reacts, or bonds, with an oxygen molecule. The reaction produces one molecule of carbon dioxide, which has the formula CO2.
Tasks