
Главная страница Случайная страница
КАТЕГОРИИ:
АвтомобилиАстрономияБиологияГеографияДом и садДругие языкиДругоеИнформатикаИсторияКультураЛитератураЛогикаМатематикаМедицинаМеталлургияМеханикаОбразованиеОхрана трудаПедагогикаПолитикаПравоПсихологияРелигияРиторикаСоциологияСпортСтроительствоТехнологияТуризмФизикаФилософияФинансыХимияЧерчениеЭкологияЭкономикаЭлектроника
Ionic bonds
|
|
An ionic bond is a type of chemical bond, in which one atom gives away/transfers its valence electron to another electron, to gain stability according to the правило октета, thus, forming oppositely charged ions. When an ionic bond is formed, a negative ion and positive ion is formed, which are attracted to each other, thereby maintaining the bond. The сила притяжения between the negative and positive ions increases as the distance between the atoms decreases. Ionic bonds are formed between metals and nonmetals, with significantly large различие электроотрицательности. Ionic bonds are weaker than covalent bonds.
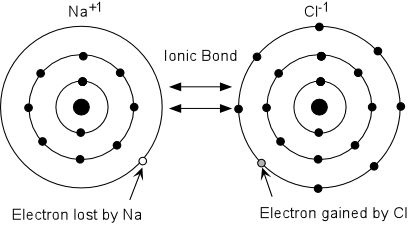 The most common and popular example of an ionic bond is that of sodium chloride or table salt. Since sodium (Na) possesses 11 electrons (2, 8, 1), with one electron in the внешней валентной оболочке. Thus, Na is able to give off one electron easily to form Na+ ion, whereby it becomes more stable as per the octet rule. Что касается хлора, it belongs to the halogen group, possesses 17 electrons (2, 8, 7), with seven electrons in its outermost shell. Thus, chlorine can easily gain an electron to form Cl- ion, whereby it becomes более стабильный as per the octet rule.
The most common and popular example of an ionic bond is that of sodium chloride or table salt. Since sodium (Na) possesses 11 electrons (2, 8, 1), with one electron in the внешней валентной оболочке. Thus, Na is able to give off one electron easily to form Na+ ion, whereby it becomes more stable as per the octet rule. Что касается хлора, it belongs to the halogen group, possesses 17 electrons (2, 8, 7), with seven electrons in its outermost shell. Thus, chlorine can easily gain an electron to form Cl- ion, whereby it becomes более стабильный as per the octet rule.
When one Na atom comes in contact with one Cl atom, the Na atom will readily give up its electron to become a more stable Na+ ion, while the Cl atom will readily accept the electron to form a more stable, Cl- ion. The resultant ions are oppositely charged, which is why they get attracted to each other, until they touch each other, forming Na+Cl- ‘molecule’. The electric attraction between Na+ and Cl- ions is ничто иное как the ionic bond. Ionic bonds are not as strong as covalent bonds, which is why we are able to break salt into smaller pieces.
7. Watch the video 'What is an Atom? '. Fill in the gaps in the following sentences.
1. All __________ is made of elements – substances that cannot be __________ by chemical activity.
2. An atom is the __________ of an element that retains the element's __________.
3. Each atom has a __________ called a nucleus.
4. The number of protons in an atom __________ an element's physical properties.
5. Electrons and protons __________ each other.
6. Individual atoms can __________ or __________ electrons.
7. Scientists call atoms with ___________ isotopes.
 8. Prepare the reports upon one of the following topics:
8. Prepare the reports upon one of the following topics:
1. Chemistry as a science.
2. Alchemy and alchemists.
3. The Russian noted chemists.
4. The twenty-first-century chemistry.
5. New frontiers in chemistry.