
Главная страница Случайная страница
КАТЕГОРИИ:
АвтомобилиАстрономияБиологияГеографияДом и садДругие языкиДругоеИнформатикаИсторияКультураЛитератураЛогикаМатематикаМедицинаМеталлургияМеханикаОбразованиеОхрана трудаПедагогикаПолитикаПравоПсихологияРелигияРиторикаСоциологияСпортСтроительствоТехнологияТуризмФизикаФилософияФинансыХимияЧерчениеЭкологияЭкономикаЭлектроника
Other reactions
|
|
Most chemical reactions take place between compounds rather than between elements or elements and compounds. In such cases, it is often possible for atoms to exchange the other atoms or groups of atoms to which they are attached. For example, there are two compounds known as sodium sulfate and barium chloride. Sodium sulfate consists of two atoms
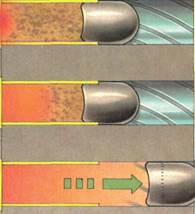
Combustion takes many forms, all of which are examples of oxidation. Burning (above/ is a comparatively slow reaction in. chemical terms, although often extremely destructive. Even the explosively fast reactions involved in firing a pistol (below! take place in stages. The purple percussion cap at the base of the yellow cartridge (A) is detonated by a hammer or firing pin (B). This ignites the pro-pellant charge, which " burns" to produce a hot gas (CI. The gas then drives the bullet out of the barrel (D).

|

|

|
|
20 Atoms, elements, and molecules: Key chemical reactions

|
 Smelting of metals (above) involves chemical reduction. This often requires large amounts of energy to " drive" the reactions. An example is the conversion of iron oxide to metallic iron. The corresponding oxidation reaction, as in the rusting of iron (above right), requires less energy. It proceeds spontaneously in moist air.
Smelting of metals (above) involves chemical reduction. This often requires large amounts of energy to " drive" the reactions. An example is the conversion of iron oxide to metallic iron. The corresponding oxidation reaction, as in the rusting of iron (above right), requires less energy. It proceeds spontaneously in moist air.
of sodium combined with a sulfate (a sulfur atom with four oxygen atoms). Barium chloride consists of an atom of barium combined with two atoms of chlorine. When these two compounds react with each other, they are said to " decompose" into two new compounds. The sulfate stays intact and attaches itself to the barium. The sodium, in turn, attaches to the chlorine and forms sodium chloride, common salt.
Such " double decomposition" reactions, as they are called, depend on the ionic character of the compounds in solution.
An atom in a normal or neutral state has the same number of electrons as it does protons. See the first article in this section for a fuller discussion of atoms. If there are more electrons than protons, the atom (called an anion) carries a negative electric charge. If there are less electrons than protons, the atom (called a cation) carries a positive electric charge. An electrically charged atom (or group of atoms) is known as an ion. The attraction of cations to anions and vice versa is very important in chemical reactions.
In the reaction involving sodium sulfate and barium chloride, the ions of each compound
split apart under the influence of water. The barium and sulfate ions combine very strongly and precipitate (leave the solution). These two qualities ensure that the reaction goes to completion. This means that the conversion of one compound into another is effectively complete.
A common type of double decomposition occurs when an acid is mixed with a base. In general, acidic compounds (acids) are those that can form hydrogen ions (positively charged hydrogen atoms) in a solution, usually of water. Bases (or alkalis, as the common ones are also called) form hydroxyl ions. These are negatively charged molecules consisting of one oxygen atom and one hydrogen atom. The acid strength of a solution is measured in terms of the concentration of hydrogen ions and is expressed as a pH number. A pH of less than 7 indicates an acid, one of greater than 7, a base.
When acids mix with bases, they are said to neutralize each other. In such acid-base neutralizations, the products are always a salt (the common name for a compound composed of ions) and water.
A reaction in which a single reactant breaks