
Главная страница Случайная страница
КАТЕГОРИИ:
АвтомобилиАстрономияБиологияГеографияДом и садДругие языкиДругоеИнформатикаИсторияКультураЛитератураЛогикаМатематикаМедицинаМеталлургияМеханикаОбразованиеОхрана трудаПедагогикаПолитикаПравоПсихологияРелигияРиторикаСоциологияСпортСтроительствоТехнологияТуризмФизикаФилософияФинансыХимияЧерчениеЭкологияЭкономикаЭлектроника
Theoretical information. Experiments have shown that the amount of heat necessary to definite temperature change of a given substance depends upon the quantity or mass of that
|
|
Experiments have shown that the amount of heat necessary to definite temperature change of a given substance depends upon the quantity or mass of that substance as well as upon the nature of the substance itself. Different materials require different quantities of heat to raise their temperature by 1oC. To describe these facts, we say that every material has its own specific heat.
The specific heat of any substance is defined as the quantity of heat required to raise the temperature of one kilogram of substance by one degree Centigrade. The corresponding table represents substances and their specific heat values. Water has higher specific heat than most other common substances. A quantity of heat is measured in Joules (J).
Units of molar quantities are obtained from specific units by replacing the mass by the quantity of substance. Therefore, the molar heat is defined as the quantity of heat necessary to raise the temperature of one mole of substance by 1oC.
The relation between molar heat and specific heat can be obtained from their definitions and looks like the following:
C = m c,
where C and c are molar and specific heats respectively; m is the molar mass of a substance.
Properties of liquids and solids are almost independent on the manner of heating. Molar and specific heats of gases depend on a condition of heating. Gas can be heated at a constant volume or at a constant pressure. Therefore we obtain molar or specific heat at a constant volume (CV, cV) or at a constant pressure (Cp, cp).
When gas is heated at a constant volume, the gas does no work. However, during the process at a constant pressure, the expanding gas produces work. Hence, this method of heating gives a higher value for molar and specific heat than the constant-volume method.
If the molar heats Cp and CV for a given gas are assumed to have the same values at all temperatures and pressures, it follows that the ratio of the two molar heats of any given gas is also a constant value. Both this ratio and the difference between the molar heats are very important constants in thermodynamics.
The molar heat dependence on heating conditions can be ascertained by the Mendeleyev-Chaperon equation (ideal gas state equation):
 . (6.1)
. (6.1)
This equation connects the volume of gas V, its pressure p, absolute temperature T, quantity of substance n (n = m/m) and gas constant R (R = 8, 31 J/mole× K).
The formula for one mole of substance can be easily obtained from equation (6.1):
 . (6.2)
. (6.2)
Then we must also use the first law of thermodynamics:
 ,
,
where dQ is the quantity of heat received by the gas; dU is a change of the gas internal energy; dA is the work performed by the gas while its volume enlarges.
In our case n = 1, so for a molar heat at constant pressure, we obtain:
 , (6.3)
, (6.3)
where dT is a temperature change.
In case of thermodynamic process at constant volume, we have:
V = const; dV = 0; dA = pdV = 0.
So molar heat at constant volume is determined by the formula
 . (6.4)
. (6.4)
Taking a derivative from formula (6.2), one can obtain the equation: pdV + Vdp = RdT.
In case of the process at constant pressure dp = 0 hence
pdV = RdT = dA.
Using the last equality and formula (4. 3), we get the following equation:
 .
.
But we have formula (6.4) then Cp = CV + R.
The last formula is called Mayer’s equation.
Thus, gas constant R is determined by the work performed by one mole of gas while temperature increases by 1 K at constant pressure.
Molecular theory states that molar heat depends on a number of degrees of freedom of molecules i:
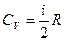 ;
;  .
.
The number of degrees of freedom of molecules is equal to the number of independent axes for determination of a molecule position in space. As for monatomic gas i = 3, we can calculate the values of molar heats:
 ;
;  .
.
Molar heats are constant for all monatomic gases. Polyatomic gas molecules have also rotary axes. Therefore, for diatomic gas i = 5 and for other polyatomic gases i = 6.
The ratio of molar heat at constant pressure to molar heat at constant volume is called adiabatic exponent g.
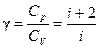 .
.
Changing gas state without heat transfer is called adiabatic process. As a rule, adiabatic processes are very fast because of inability to complete isolating any system.
The pressure and volume of gas are connected in adiabatic process by Poisson’s equation:
pV g = const, i.e. 
Poisson’s equation can also be written as follows:
 or
or  .
.