
Главная страница Случайная страница
КАТЕГОРИИ:
АвтомобилиАстрономияБиологияГеографияДом и садДругие языкиДругоеИнформатикаИсторияКультураЛитератураЛогикаМатематикаМедицинаМеталлургияМеханикаОбразованиеОхрана трудаПедагогикаПолитикаПравоПсихологияРелигияРиторикаСоциологияСпортСтроительствоТехнологияТуризмФизикаФилософияФинансыХимияЧерчениеЭкологияЭкономикаЭлектроника
Measurement procedure and equipment
|
|
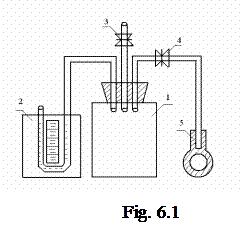
The equipment (Fig. 6.1) represents glass container 1 of enough capacity. By means of taps 3 and 4, the container is connected with both the air and pump 5. A rubber pipe joins the container with liquid manometer 2.
Let us open tap 3. Because of that, the pressure in the container becomes equal to the atmospheric one. Then we raise pressure in the container by means of the pump and close the tap. This action gives the temperature rise. After that, the temperature of the gas in the container lowers up to the temperature of the air and the difference between the liquid levels in the manometer tubes diminishes.
Thus, we should wait for some minutes until the liquid levels stop changing. The difference between the liquid levels of the manometer will be marked as h 1. If then we turn on tap 3 for some seconds, the gas in the container will get the atmospheric pressure again. However, as the pressure in the container lowers, the temperature decreases. As the process goes fast without heat transfer, cooling can be called adiabatic process. The following increase of the gas pressure in the container is connected with warming the gas up to the air temperature. This process goes at the constant volume.
Thus, we have three gas states:
 1st state – temperature T 0 , pressure p a+ h 1;
1st state – temperature T 0 , pressure p a+ h 1;
2nd state – temperature T 2, pressure p a;
3rd state – temperature T 0 , pressure p a+ h 2,
where T 0 is the air temperature; p a is the atmospheric pressure measured in millimetres of water (pa»104 mm).
Passing from the first state to the second one is an adiabatic process and passing from the second state to the third one is the process at a constant volume. These processes are described by the following equations:
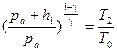 – for the adiabatic process; (6.5)
– for the adiabatic process; (6.5)
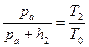 – for the process at constant volume. (6.6)
– for the process at constant volume. (6.6)
From equations (6.5) and (6.6), we obtain:
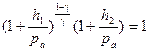 .
.
Taking the logarithm of the last equation, we get:
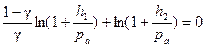 . (6.7)
. (6.7)
According to the experiment conditions:
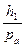 < < 1 and
< < 1 and 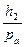 < < 1.
< < 1.
Thus, it is possible to simplify equation (6.7).
As ln(1+ x) = x (while x < < 1), then
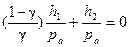 .
.
Thus, the expression for adiabatic exponent determination:
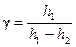 . (6.8)
. (6.8)