
Главная страница Случайная страница
КАТЕГОРИИ:
АвтомобилиАстрономияБиологияГеографияДом и садДругие языкиДругоеИнформатикаИсторияКультураЛитератураЛогикаМатематикаМедицинаМеталлургияМеханикаОбразованиеОхрана трудаПедагогикаПолитикаПравоПсихологияРелигияРиторикаСоциологияСпортСтроительствоТехнологияТуризмФизикаФилософияФинансыХимияЧерчениеЭкологияЭкономикаЭлектроника
Major Clinical Syndromes. Cardiac Rhythm Disorders (Arrhythmias)
|
|
Cardiac Rhythm Disorders (Arrhythmias)
Any deviations from the normal rhythm of the heart are called ar
rhythmias. These imply alterations in the heart rate, in succession or force
of heart contractions, and also changes in the sequence of excitation and
contraction of the atria and ventricles. Most arrhythmias are connected
with functional changes or anatomical affections of the heart's conduction
system. ■.... ■ ■ *<.', ■. _-.,.............:.».-...«■,
Under normal conditions, the sino-atrial node has the highest automaticity and is therefore the pacemaker of the cardiac rhythm. Impulses are generated in the sino-atrial node at regular intervals (from 60 to 70 beats per min). The impulses are transmitted from the sino-atrial node (Wenckebach, Bachman, Thorel bundles) to the atrioventricular node at a rate of 0.8-1 m/s. This rate sharply decreases in the region of the atrioventricular node (to 0.05 m/s) and the atrial systole therefore ends earlier than the excitation spreads over onto the myocardium of the ventricles to cause their contraction. Impulses are trasmitted from the atrioventricular node through the His bundle at a higher rate (1-1.5 m/s), while the rate of impulse propagation in Purkinje's fibres is as high as 3—4 m/s. Excitation is the triggering mechanism for the heart contraction. During the heart contraction, and immediately after systole, the cardiac muscle is absolutely refractory; then its excitability gradually restores.
Automaticity is characteristic of the entire conduction system of the heart, but in normal conditions it is inhibited by the high activity of the sino-atrial node, which is the automaticity centre of the first order. If the sino-atrial node is affected, or the transmission of excitation to the atrioventricular node is impaired, the atrioventricular node becomes the pacemaker (the second-order automaticity centre). Impulses are generated here at a rate of 40 to 50 per min. If the His bundle is affected, the impulses causing contraction of the heart may be generated in the Purkinje fibres (automaticity centre of the third order), but the rate of the cardiac rhythm then slows down to 20-30 beats per min.
The normal cardiac rhythm may change (1) in affected automaticity of the sino-atrial node, when the rate or sequence of impulses is altered; (2) in development of a focus of increased activity in the myocardium, that can generate impulses to initiate heart contractions apart from their generation in the sino-atrial node (ectopic arrhythmia); (3) in disordered conduction of the impulses from the atria to the ventricles or inside the ventricles themselves. Abnormal rhythm can also be due to impaired contractility of the myocardium. Arrhythmia can sometimes depend on changes in several functions of the heart such as automaticity, excitability, conduction or contractility.
Arrhythmias associated with altered automaticity of the sino-atrial node (sinus arrhythmia). When automaticity of the sino-atrial node is upset, the rate of impulse generation may either accelerate (sinus tachycardia) or slow down (sinus bradycardia), or the sequence of impulses may be changed with their generation at irregular intervals (sinus arrhythmia).
Sinus tachycardia is directly connected with effects of biologically active substances which increase excitability of the sino-atrial node. This phenomenon may also depend on the change in the tone of the vegetative nervous system. It develops with intensified effect of the sympathetic nervous system. The rate of cardiac contractions in sinus tachycardia usually varies from 90 to 120 and sometimes to 150-160 per min. Sinus tachycardia develops during meals, physical exertion and emotional stress. At elevated body temperature, the heart rate increases by 8—10 per min per each degree over 37 °C. Sinus tachycardia is a frequent symptom of myocarditis, heart defects, and other diseases. It develops by reflex
17-1556

 Special Part
Special Part
JLi
| R Q S |
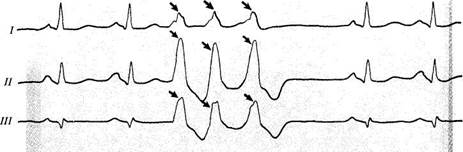
Fig. 51. Sinus arrhythmia.
a—sinus tachycardia (110 b.p.m.); b— pronounced sinus bradycardia (34 b.p.m.); c—sinus arrhythmia. Cardiac complexes last for 0.70, 0.94, 0.82, and 0.86 s.
mechanism in heart failure and in response to the increased pressure in the orifices of venae cavae. Tachycardia often develops in neurosis, anaemia, hypotension, and in many infectious diseases and toxicosis; it can be provoked by some pharmacological preparations (adrenaline, caffeine, atropine sulphate, etc.), and in thyrotoxicosis.
The clinical signs of sinus tachycardia are heart palpitation and accelerated pulse. The T-P interval on ECG shortens and the P wave may interpose on the T wave (Fig. 51a).
Sinus bradycardia is connected with slowed excitation of the sino-atrial node, which in turn depends mostly on the increased influence of the parasympathetic nervous system on the heart (or decreased influence of the sympathetic nervous system). Automaticity of the sino-atrial node decreases in sclerotic affections of the myocardium and in the cold. The cardiac rate in sinus bradycardia decreases to 50-40 (in rare cases to 30) beats per min. Bradycardia may occur in well-trained athletes. It is not permanent and the heart rhythm is accelerated during exercise as distinct from pathological bradycardia in atrioventricular block when bradycardia persists during and after exercise. If automaticity of the sino-atrial node sharply decreases (sick-sinus syndrome), the second- or third-order centres may function as the pacemaker, i.e. ectopic arrhythmias develop (see below).
Sinus bradycardia occurs in increased intracranial pressure (tumour and oedema of the brain, meningitis, cerebral haemorrhage), in myx-
Chapter 6. Blood Circulatory System
oedema, typhoid fever, jaundice, starvation, lead and nicotine poisoning, and due to effect of quinine and digitalis preparations. It may develop by reflex during stimulation of baroreceptors of the carotid sinus and the aortic arch in essential hypertension, and can be provoked by pressure on the eye-ball (Dagnini-Aschner reflex), or by irritation of receptors of the peritoneum and the internal organs.
Mild bradycardia is not attended by any subjective disorders, nor does it produce any effect on the circulation. Marked bradycardia (under 40 beats per min) may cause nausea and loss of consciousness due to cerebral anaemia. Objective examination reveals slow pulse. The ECG in sinus bradycardia (Fig. 5lb) reveals the unchanged atrial or ventricular complexes; the T-P interval only increases to show protraction of electrical diastole of the heart; the P-Q interval sometimes increases insignificantly (to 0.20-0.21 s).
Sinus arrhythmia. Sinus arrhythmia characterized by irregular generation of impulses is due to variations in the tone of the vagus. It would commonly be associated with respiratory phases (respiratory arrhythmia): the cardiac rhythm accelerates during inspiration and slows down during expiration. Sinus arrhythmia is observed in children and adolescents (juvenile arrhythmia), in patients convalescing from infectious diseases, and in certain diseases of the central nervous system. It can be a sign of pathology in rare cases when arrhythmia is not connected with respiration or when it develops in the aged during normal respiration.
Clinically sinus arrhythmia is not attended by any subjective disorders. The cardiac rhythm and pulse rate only change with respiratory phases, and the intervals between the heart complexes (R-R intervals) vary in length on the ECG (Fig. 51c).
Ectopic arrhythmias. Additional (heterotopic or ectopic) foci of excitation can arise at any site of the conduction system (in the atria, ventricles, atrioventricular region). They can cause premature contraction of the heart before termination of the normal diastolic pause. This premature contraction is called extrasystole, and the disorder of the cardiac rhythm is called extrasystolic arrhythmia. If the activity of the ectopic focus is very high, it can become a temporary pacemaker, and all impulses governing the heart will during this time be emitted from this focus. The cardiac rhythm is then markedly accelerated. The condition is known as paroxysmal tachycardia.-Ectopic arrhythmias are often due to increased excitability of the myocardium.
The phenomenon known as re-entry can be another mechanism of ectopic arrhythmia. If an impulse meets an obstacle in the pathway of its conduction (local conduction disorder), the excitation wave can return from this obstacle to excite the myocardium.
17*
Special Part
Chapter 6. Blood Circulatory System
 Extrasystolic arrhythmia. Extrasystole usually develops during normal contractions of the heart governed by the sino-atrial node (nomotopic contractions). Ectopic foci of excitation can arise at any site of the conduction system. Usually excitations arise in the ventricles, less frequently in the atria, the atrioventricular node, and in the sino-atrial node (sinus extrasystole). A nomotopic contraction of the heart that follows extrasystole occurs in a longer (than normal) lapse of time. This can be explained as follows. During the atrial extrasystole, excitation from the ectopic focus is transmitted to the sino-atrial node to " discharge" it, as it were. The next impulse arises in the sino-atrial node only in a lapse of time that is required to " discharge" the node and to form a new impulse.
Extrasystolic arrhythmia. Extrasystole usually develops during normal contractions of the heart governed by the sino-atrial node (nomotopic contractions). Ectopic foci of excitation can arise at any site of the conduction system. Usually excitations arise in the ventricles, less frequently in the atria, the atrioventricular node, and in the sino-atrial node (sinus extrasystole). A nomotopic contraction of the heart that follows extrasystole occurs in a longer (than normal) lapse of time. This can be explained as follows. During the atrial extrasystole, excitation from the ectopic focus is transmitted to the sino-atrial node to " discharge" it, as it were. The next impulse arises in the sino-atrial node only in a lapse of time that is required to " discharge" the node and to form a new impulse.
In ventricular extrasystole, the time between the extrasystolic contraction and subsequent nomotopic contraction is even longer. The impulse from the heterotopic focus, located in the ventricles, propagates only over the ventricular myocardium; it would not be usually propagated to the atria via Aschoff-Tawara node. The impulse occurs in normal time in the sino-atrial node but it is not transmitted to the ventricles because they are refractory after the extrasystolic excitation. The next impulse from the sino-atrial node will only excite and contract the atria and the ventricles. A long " compensatory" pause therefore follows the ventricular extrasystole which lasts till the next nomotopic contraction.
Extrasystolic arrhythmia is quite common. It may occur in practically healthy individuals as a result of overexcitation of certain sites of the conduction system due to the action of the extracardiac nervous system in heavy smokers and in persons abusing strong tea or coffee; it can occur by reflex in diseases of the abdominal organs. Extrasystole often attends various cardiovascular pathological conditions due to inflammatory or dystrophic affections of the myocardium or its deficient blood supply; or it may be due to hormonal disorders (thyrotoxicosis, menopause), various intoxications, disorders of electrolyte metabolism, etc.
Patients with extrasystole can feel their heart missing a beat (escape beat) and a subsequent strong stroke. Auscultation of the heart reveals its premature contraction with a specific loud first sound (due to a small diastolic filling of the ventricles). Extrasystole can be easily revealed by feeling the pulse: a premature weaker pulse wave and a subsequent long pause are characteristic. If extrasystole follows immediately a regular contraction, the left ventricle may be filled with blood very poorly and the pressure inside it may be so small that the aortic valve would not open during the extrasystolic contraction and the blood will not be ejected into the aorta. The pulse wave on the radial artery will not be then detectable (missing pulse). The ECG of all extrasystoles are characterized by: (1) premature appearance of the cardiac complex; (2) elongated pause between the ex-
trasystolic and subsequent normal contraction. According to the site of origin, extrasystoles are classified as atrial and atrioventricular (nodal), which are given a common name of supraventricular, and also ventricular (left- and right-ventricular) extrasystoles.
Excitation of the atria only changes in atrial extrasystole because the impulse is generated not in the sino-atrial node, and the ventricles are excited by the usual way. The ECG of atrial extrasystole is characterized by the following signs (Fig. 52a): (1) premature appearance of the cardiac complex; (2) preservation of the atrial P wave which may be slightly disfigured and superimposed on the preceding T wave; this depends on the abnormal atrial excitation from a heterotopic focus; (3) normal shape of the ventricular complex; (4) slight elongation of the diastolic pause (T-P interval) following the extrasystolic contraction.
In atrioventricular (nodal) extrasystole the excitation of the atria differs from normal more substantially than in atrial extrasystole. The Aschoff-Tawara node impulse is transmitted to the atria retrogradely, from bottom to top. The ventricles are excited in nodal extrasystole in the usual way. The following signs are characteristic of the ECG in nodal extrasystole: (1) premature appearance of the cardiac complex; (2) changes in the P wave which becomes negative to show the retrograde atrial excitation (in some cases the P wave is absent on the ECG); (3) the position of the P wave with respect to the ventricular complex changes, which depends on the rate of

Fig. 52. Extrasystole. a—atrial; b —nodal; c —ventricular; d —polytopic. Extrasystoles are marked by the arrows (for details see
the text).
Special Part
Chapter 6. Blood Circulatory System
propagation of the excitation wave onto the atria and the ventricles. If excitation of the atria is followed by excitation of the ventricles, the negative P wave is recorded before the QRS complex; if the ventricles are excited first, the negative P wave follows the QRS complex. If the atria and ventricles are excited synchronously, the P wave is not recorded separately but superimposes the QRS complex to alter somewhat its configuration (see Fig. 53). In other cases, the configuration of the ventricular complex in nodal extrasystole usually does not change; the diastolic pause becomes longer.

|
| Qs |
| Fig. 53. Nodal extrasystoles. a —from the upper part of the sino-atrial node; b —from the lower part of the node. |
Heart excitation order changes sharply in ventricular extrasystole. First, the ventricular impulse is not usually transmitted retrogradely through the Aschoff-Tawara node and the atria are not therefore excited. Second, the ventricles are not excited synchronously (as in normal cases), but one after another, i.e. that ventricle is excited first where the ectopic focus is located. The time of excitation of the ventricles is therefore longer and the QRS complex wider. The ECG is characterized by the following signs: (1) premature appearance of the ventricular complex; (2) absence of the atrial P wave; (3) deformation of the QRS complex due to its increased voltage and length; (4) since the sequence of relaxation in the ventricles changes, the shape and the height of the T wave changes as well. As a rule, the T wave is enlarged and its direction is opposite to that of the maximum wave of the QRS complex (the T wave is negative if the R wave is high, and positive if the S wave is deep). The ventricular extrasystole is followed by a long (full) compensatory pause (except in interpolated extrasystoles): the atria are only excited by the sinus impulse that follows the extrasystole because the ventricles are refractory at this moment. The P wave corresponding to the atrial excitation is " lost" in the disfigured extrasystolic ventricular complex. Only next (second to the extrasystole) sinus impulse

Fig. 54. Left-ventricular extrasystole.
excites both the atria and the ventricles, while the ECG shows a normal cardiac complex.
Sometimes it is possible to determine in which particular ventricle the ectopic focus is located. This can be done from the configuration of the ventricular complex in various ECG leads. Left-ventricular extrasystole is characterized by a high R wave in the third standard lead and the deep S wave in the first lead (Fig. 54). In right-ventricular extrasystole, the ex-
Special Part
Chapter 6. Blood Circulatory System
 | ||||
 | ||||
 | ||||

|
|

|

|
| Fig. 57. Ventricular trigeminy. |
| V |
Fig. 55. Right-ventricular extrasystole.
Fig. 56. Ventricular bigeminy.
Fig. 58. Group extrasystole. Two normal cardiac complexes are followed by three ventricular
extrasystoles.
trasystolic complex is characterized by a high R wave in the first lead, and a deep S wave in the third lead (see Fig. 55).
Chest leads are very important for the topic diagnosis of ventricular extrasystole. Left-ventricular extrasystoles are characterized by the appearance of the extrasystolic complex with a high R wave in the right chest leads and a broad or deep S wave in the left chest leads. In right-ventricular extrasystole, on the contrary, the deep S wave is recorded in the right chest leads, and a high S wave in the left chest leads. If excitability of the myocardium is high, several (rather than one) ectopic foci may exist. Extrasystoles generated in various heart chambers and having different configuration then appear on the ECG {polytopic extrasystole).
Wherever an ectopic focus may arise, its impulses may alternate in a certain order with the normal impulses of the sino-atrial node. This phenomenon is known as allorhythmia. Extrasystole may alternate with each sinus impulse (bigeminy; see Fig. 56), or it may follow two normal impulses (trigeminy; see Fig. 57), or three normal impulses (quadrigeminy), etc. If the heterotopic focus is even more active, a normal contraction may be followed by several extrasystoles at a run (group extrasystole; Fig. 58), which sometimes precedes an attack of paroxysmal tachycardia.
Paroxysmal tachycardia. This is a sudden acceleration of the cardiac rhythm (to 180-240 beats per min). At attack of paroxysmal tachycardia
Special Part
Chapter 6. Blood Circulatory System
 | |||
 | |||

|
| JLAJLJ |
may last from several seconds to a few days and terminate just as unexpectedly as it begins. During an attack, all impulses arise from a heterotopic focus because its high activity inhibits the activity of the sino-atrial node. Paroxysmal tachycardia (like extrasystole) may occur in subjects with increased nervous excitability, in the absence of pronounced affections of the heart muscle, but it arises more likely in the presence of a severe heart disease (e.g. myocardial infarction, heart defects or car-diosclerosis).
During an attack of paroxysmal tachycardia, the patient feels strong palpitation, discomfort in the chest, and weakness. The skin turns pale, and if attack persists, cyanosis develops. Paroxysmal tachycardia is characterized by swelling and pulsation of the neck veins, because during accelerated pulse (to 180-200 per min) the atria begin contracting before the ventricular systole ends. The blood is ejected back to the veins from the atria to cause pulsation of the jugular veins. Auscultation of the heart during an attack of paroxysmal tachycardia reveals decreased diastolic pause, whose length nears that of the systolic one, and the heart rhythm becomes foetal (pendulum). The first sound increases due to insufficient ventricular diastolic filling. The pulse is rhythmic, very fast, and small. Arterial pressure may fall. If an attack persists (especially if it develops in the presence of a heart disease) symptoms of cardiac insufficiency develop.
Like in extrasystole, the heterotopic focus in paroxysmal tachycardia may be located in the atria, the atrioventricular node, and the ventricles. It is possible to locate the focus only by electrocardiography: a series of ex-trasystoles follow on an ECG at regular intervals and at a very fast rate. Figure 59a shows the ECG taken during an attack of supraventricular paroxysmal tachycardia (the P wave cannot be seen because of accelerated cardiac rhythm and the shape of the ventricular complex is not changed); an ECG which follows next is taken in a patient with ventricular tachycardia; it shows a series of altered and broadened ventricular complexes (similar to those in ventricular extrasystoles).
Arrhythmias due to disordered myocardial conduction. Transmission of the impulse may be blocked at any part of the heart's conduction system. The following types of heart blocks are distinguished: (1) sino-atrial block, in which beats are sometimes missing in the sino-atrial node and the impulse is not transmitted to the atria; (2) intra-atrial block, in which transmission of excitation through the atrial myocardium is impaired; (3) atrioventricular block, in which conduction of impulses from the atria to the ventricles is impaired; (4) intraventricular block, in which conduction of impulses through the His bundle and its branches is impaired.
Block may develop in inflammatory, dystrophic, and sclerotic affec-
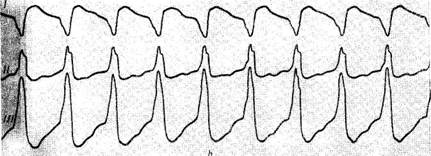
Fig. 59. Paroxysmal tachycardia.
a—supraventricular paroxysmal tachycardia (170 b.p.m.); b— ventricular paroxysmal tachycardia (170
b.p.m.).
tions of the myocardium (e.g. rheumatic and diphtheritic myocarditis or cardiosclerosis). The conduction system may be affected by granulomas, cicatrices, toxins, etc. Conduction is often impaired in disordered coronary circulation, especially in myocardial infarction (the interventricular septum is involved). Block may be persistent and intermittent. Persistent block is usually connected with anatomic changes in the conduction system, whereas intermittent block depends largely on the functional condition of the atrioventricular node and the His bundle and is often connected with increased influence of the parasympathetic nervous system; atropine sulphate is an effective means that restores conduction.
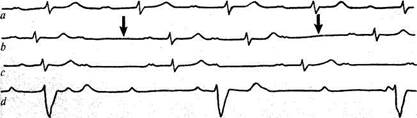
Clinical signs of block depend on its location. Sino-atrial block is characterized by periodic missing of the heart beat and pulse beat. The ECG (Fig. 60) shows periodic missing of the heart complex in the presence of a regular sinus rhythm (neither P wave nor the QRST complex are recorded); the length of diastole doubles.
Intra-atrial block can only be detected electrocardiographically because clinical signs are absent. Figure 61 shows the ECG with altered P waves;
Special Part
Chapter 6. Blood Circulatory System
 |
|

Fig. 60. Sino-atrial block. The third cardiac complex is followed by a pause equal to two
preceding R-R intervals.
 since the time of atrial excitation increases, the length of the P waves increases as well (to 0.1 s).
since the time of atrial excitation increases, the length of the P waves increases as well (to 0.1 s).
| Fig. 61. Intra-atrial block. P waves are broadened (P = 0.14 s) and serrated; the P wave in the first chest lead has two phases. |
Atrioventricular block is most important clinically. It is classified into three degrees by gravity. The first degree can only be revealed electrocar-diographically (Fig. 62a) by the increased P-Q interval (to 0.3-0.4 s and more). This block cannot be detected clinically, except that splitting of the first sound may sometimes be detected by auscultation (splitting of the atrial component). The second-degree atrioventricular block is characterized by dualism of its signs. Conduction of the Aschoff-Tawara node and His bundle is impaired: each impulse transmitted from the atria to the ventricles increases and the P-Q interval on the ECG becomes longer with each successive beat. A moment arrives at which one impulse does not reach the ventricles and they do not contract, hence the missing QRS complex on an ECG (Fig. 62b). During a long diastole, which now follows, the conduction power of the atrioventricular system is restored, and next impulses will again be transmitted, but their gradual slowing down will be noted again; the length of the P-Q interval will again increase in each successive complex (Fig. 626). The length of diastole which follows the P wave is called the
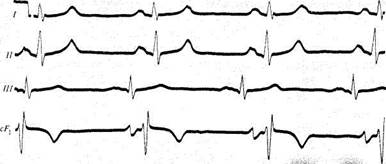
Fig. 62. Atrioventricular block.
a —I degree (the P-Q interval in all cardiac complexes is 0.40 s); b —Ha degree with Samoilov-Wenckebach periods (marked by arrows); the P-Q interval in the first cardiac complex is 0.36 s, then follows the P wave; the ventricular complex is not recorded after the P wave; the next P-Q interval is 0.28 s and then 0.38 s; the next P wave is again followed by the Samoilov-Wenckebach period; c —lib degree block with the ratio of 2: 1; the atrial rhythm is 84 cp.m., and the ventricular rhythm is 42 cp.m.; d —complete heart block; atrial rhythm is 85 cp.m.; the ventricular rhythm 20 cp.m.
Samoilov- Wenckebach period. This type of block is characterized clinically by periodically missing ventricular contractions, and hence missing pulse beats, which correspond to the Samoilov-Wenckebach period. On the other hand, the second-degree atrioventricular block can be characterized by a worse conduction. The P-Q interval remains constant, but only each second, third, or (less frequently) fourth impulse is transmitted to the ventricles. The number of P waves on the ECG is therefore larger than of ventricular complexes (see Fig. 62c). This is known as incomplete heart block with a 2: 1, 3: 1, etc. ratio. Considerable deceleration of the ventricular rhythm and slow pulse are characteristic, especially in 2: 1 block. If each third or fourth beat is missing, the pulse is irregular and resembles trigeminy or quadrigeminy with early extrasystoles and pulse deficit. If the heart rhythm slows down significantly, the patient may complain of giddiness, everything going black before his eyes, and transient loss of consciousness due to anaemia of the brain. The third-degree atrioventricular block is called complete heart block. Atrial impulses do not reach the ventricles and the sino-atrial node becomes the only pacemaker for the atria. The ventricles contract by their own automaticity in the centres of the second or third order. The number of their contractions in complete heart block is about 30—40 per min, and ventricular rhythm slows down with the lower position of the pacemaker in the conduction system.
The ECG in complete heart block is characterized by the following signs: (1) atrial P waves and ventricular complexes are recorded independently of each other, and part of the P waves may superimpose the
Special Part
Chapter 6. Blood Circulatory System
 QRS complex and become invisible on the ECG; (2) the number of ventricular complexes is usually much smaller than the number of atrial waves; (3) if the pacemaker arises from the Aschoff-Tawara node or His bundle, the shape of the ventricular complex does not change substantially; with lower location of the pacemaker in the conduction system, the QRST complexes are altered because the process of ventricular excitation is upset.
QRS complex and become invisible on the ECG; (2) the number of ventricular complexes is usually much smaller than the number of atrial waves; (3) if the pacemaker arises from the Aschoff-Tawara node or His bundle, the shape of the ventricular complex does not change substantially; with lower location of the pacemaker in the conduction system, the QRST complexes are altered because the process of ventricular excitation is upset.
| Fig. 63. Block of the left branch of the His bundle (time of intraventricular conduction is 0.17 s). |
The heart rate in persistent complete heart block may be sufficiently high (40-50 beats/min) but the patient may be unaware of the disease for a long time. Examination of such patients reveals slow, rhythmic, and full pulse. The heart sounds are dulled but a loud first sound (" pistol-shot" sound according to Strazhesko) may be heard periodically. It occurs due to coincidence of the atrial and ventricular contractions. If the ventricular rhythm slows down significantly (to 20 beats/min and less), or the heart misses a beat when incomplete heart block converts into a complete one, i.e. when the impulses from the atria are not conducted to the ventricles, while their automaticity has not yet developed, attacks (the Morgagni-Adams-Stokes syndrome) may occur due to disordered blood supply, mainly to the central nervous system. During an attack the patient loses consciousness, falls, general epileptiform convulsions develop, the respiration becomes deep, the skin pallid, the pulse very slow or even impalpable.

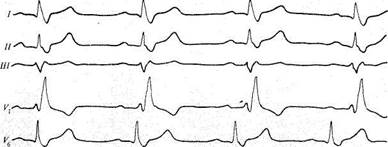
Fig. 64. Block of the right branch of the His bundle (time of intraventricular conduction is
0.15 s).
When the ventricular automaticity restores, the patient regains his consciousness and all other signs of the syndrome disappear. If automaticity is not restored for a time, fatal outcome is possible.
Intraventricular block usually develops as the right or left bundle-branch block. The left limb of the His bundle ramifies almost immediately to give left anterior and left posterior branches. Only one branch can therefore be blocked. Block of the right limb may be combined with block of the branches of the left limb. In complete block of either of the limbs, the impulse from the sino-atrial node is normally conducted through the Aschoff-Tawara node and the main part of the His bundle to meet an obstacle to its conduction in that ventricle whose branch is affected. The ventricle with the intact branch is therefore first excited and excitation is transmitted to the ventricle with the affected branch. The ventricles are thus excited slowly and in an unusual way.
The bundle-branch block is characterized electrocardiographically as follows (Figs. 63 and 64): (1) the P wave does not change; (2) the ventricles contract rhythmically by the impulse from the sino-atrial node, but since the order of the ventricular excitation is upset, markedly altered and broadened QRS complexes (which resemble complexes in ventricular ex-trasystole) are recorded; (3) the time of intraventricular conduction increases to 0.12—0.18 s and more. The shape of the ventricular complexes depends on the particular bundle branch which is blocked. If the left branch is blocked, its excitation is delayed and the ventricular complexes acquire the shape of the right-ventricular extrasystolic complexes (Fig. 63), i.e. the QRS complex broadens and changes in its shape, the S-T interval shifts, and the direction of the T waves changes to the opposite with respect
Special Part
Chapter 6. Blood Circulatory System



 to the direction of the maximum wave of the QRS complex. If the right branch of the bundle is affected, the shape of the ventricular complexes resembles that of left-ventricular extrasystoles (Fig. 64). Bundle-branch block can only be detected electrocardiographically. It has no subjective signs.
to the direction of the maximum wave of the QRS complex. If the right branch of the bundle is affected, the shape of the ventricular complexes resembles that of left-ventricular extrasystoles (Fig. 64). Bundle-branch block can only be detected electrocardiographically. It has no subjective signs.
Reduplication or splitting of the heart sounds can sometimes be auscultated. These are due to asynchronous contractions of the ventricles.
Atrial and ventricular flutter and fibrillation. Fibrillation is otherwise known as complete or absolute arrhythmia. It arises in cases with suddenly increased excitation of the myocardium and simultaneous conduction disorders. The sino-atrial node fails to function as the pacemaker and many ectopic excitation foci (to 600-800 per min) arise in the atrial myocardium, which becomes only possible with a marked shortening of the refractory period. Since conduction of these impulses is difficult, each of them only excites and causes contraction of separate muscular fibres rather than the entire atrium. As a result, minor contractions develop in the atrium (atrial fibrillation) instead of adequate atrial systole. The mechanism of fibrillation is not fully understood. It is believed that permanent circulation of the circular excitation wave in the atria can account for the development of this disorder. Only part of the impulses are transmitted to the ventricles through the Aschoff-Tawara node. Since conduction of atrial impulses is irregular, the ventricles contract at irregular intervals to cause complete arrhythmia of the pulse. Depending on the conductability of the Aschoff-Tawara node, three forms of atrial fibrillation are distinguished: tachyarrhythmic, in which ventricles contract at a rate from 120 to 160 per min, bradyarrhythmic, in which the heart rate does not exceed 60 per min, and normosystolic, in which the ventricles contract at a rate of 60-80 per min.
Fibrillation is characteristic of mitral heart diseases (especially of mitral stenosis), coronary atherosclerosis, thyrotoxicosis, etc. Fibrillation may occur as a permanent symptom or in attacks of tachyarrhythmia. Clinically fibrillation (bradyarrhythmia) may cause no subjective symptoms. Tachyarrhythmia is usually characterized by palpitation. Examination of the heart reveals complete irregularity of the heart contractions. Variations in the length of diastole account for variations in ventricular filling and hence in the intensity of the heart sounds. The pulse is also arrhythmic, pulse waves vary in height (irregular pulse), and pulse deficit often develops in frequent heart contractions. The ECG of a patient with fibrillation (Fig. 65) shows the following changes: (1) theP wave disappears; (2) multiple small waves appear which are designated by the letter T; (3) ventricular complexes follow at irregular intervals, their shape does not change substantially.
| Lj^i^ |
 ju.— i
ju.— i
Fig. 65. Fibrillation. Ventricular complexes follow on the ECG at irregular intervals; P waves are absent; small waves (/) are recorded instead.
 Atrial flutter is the upset cardiac rhythm, which nears in its pathogenesis to fibrillation. As distinct from fibrillation, the number of impulses arising in fluttering atria does not usually exceed 250—300 per min, and their conduction through the Aschoff-Tawara node is usually rhythmic. As a rule, not all atrial impulses are conducted to the ventricles. Each other, third or fourth impulse, is only conducted to the ventricles since partial (incomplete) atrioventricular block develops simultaneously. Conduction of the Aschoff-Tawara node sometimes constantly changes: each other impulse is now conducted; then the rhythm changes to conduction of each third impulse, and the ventricles contract arrhythmically. Like fibrillation, atrial flutter occurs in mitral defects, coronary atherosclerosis, and thyrotoxicosis; flutter sometimes develops in poisoning with quinine or digitalis.
Atrial flutter is the upset cardiac rhythm, which nears in its pathogenesis to fibrillation. As distinct from fibrillation, the number of impulses arising in fluttering atria does not usually exceed 250—300 per min, and their conduction through the Aschoff-Tawara node is usually rhythmic. As a rule, not all atrial impulses are conducted to the ventricles. Each other, third or fourth impulse, is only conducted to the ventricles since partial (incomplete) atrioventricular block develops simultaneously. Conduction of the Aschoff-Tawara node sometimes constantly changes: each other impulse is now conducted; then the rhythm changes to conduction of each third impulse, and the ventricles contract arrhythmically. Like fibrillation, atrial flutter occurs in mitral defects, coronary atherosclerosis, and thyrotoxicosis; flutter sometimes develops in poisoning with quinine or digitalis.
Patients with accelerated heart rate (high conduction of the Aschoff-Tawara node) complain of palpitation. Examination reveals tachycardia that does not depend on the posture of the patient, exercise or psychic strain, since the sino-atrial node does not function as the pacemaker in atrial flutter (being governed by extracardial nerves). Heart contractions are arrhythmic in patients with varying conduction of the Aschoff-Tawara node. The ECG shows high waves (Fig. 66) instead of the normal atrial P waves. The number of high waves preceding each ventricular complex depends on the conduction of the Aschoff-Tawara node.
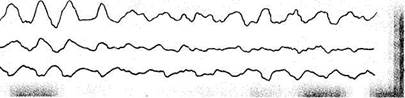
Ventricular fibrillation and flutter are gross disorders of the heart rhythm. The absence of adequate ventricular systole and contraction" of separate ventricular muscles cause pronounced disorders in the haemodynamics and rapidly lead to death. Ventricular fibrillation and flutter occur in grave affections of the myocardium (diffuse myocardial infarction, etc.). The patient loses consciousness, becomes pallid, the pulse and arterial pressure become indeterminable. The ECG shows abnormal complexes on which separate waves are distinguished with difficulty (Fig. 67).
18-1556
Special Part
Chapter 6. Blood Circulatory System 275



Fig. 66. Atria! flutter. High atrial waves are seen on the ECG.
|
| Fig. 67. Ventricular flutter and fibrillation. |
Treatment of cardiac arrhythmia includes the following measures: (1) management of the diseases which caused arrhythmia (myocarditis, ischaemic heart disease, neurosis, hyperthyroidism, etc.); (2) using means to restore ionic equilibrium in the myocardium and improve metabolism (potassium salts, vitamins, ATP, etc.); (3) in cases with increased excitability of the myocardium and in ectopic arrhythmias the following preparations are recommended: quinidine, novocainamide, aimaline, beta-adrenergic blocking agents (/3-blockers), etc.; (4) progressive ventricular fibrillation is managed by electric defibrillation, i.e. short (0.01 s) single electric discharge of 5000-7000 V, which causes instantaneous excitation of all parts of the myocardium and restores the normal cardiac rhythm. Defibrillation is managed by an apparatus known as a defibrillator. Its two electrodes are attached to the chest (one below the left scapula and the other on the heart, or one below the right clavicle and the other over the heart apex). Electric impulses are also given to treat permanent fibrillation or paroxysmal tachycardia if medicamentous therapy proves inefficient; (5) electric stimulation of the heart (artificial pacemaker) is also indicated in stoppage of the heart, in pronounced bradycardia, in complete atrioven-tricular block.
Circulatory Insufficiency '
This is a pathological condition in which the cardiovascular system fails to supply the necessary amount of blood to the organs and tissues for their adequate function. This condition arises due to affection of the heart or of the vessels alone, or it may be secondary to general disorders of the cardiovascular system. The clinic of circulatory insufficiency is usually associated with heart failure in which the function of the entire circulatory apparatus soon becomes affected.
Heart failure is associated with decreased contractility of the myocardium. The amount of venous blood flowing to the heart and the resistance, which the myocardium has to overcome in order to eject blood into the vessels, exceeds the power of the heart to handle the blood flowing from the veins into the arteries. The numerous causes of heart failure can be classified into the following two major groups.
1. Heart failure caused by diseases which affect primarily the myocar
dium and its metabolism. This condition arises in (a) infectious, inflam
matory, and toxic affections of the myocardium (myocarditis of various
aetiology, intoxication of the myocardium with alcohol, narcotic drugs,
and other poisons); (b) insufficient blood supply to the myocardium
(disordered coronary circulation, anaemia); (c) metabolic disorders,
avitaminosis, and endocrine dysfunction.
2. Heart failure due to overloading or overstrain of the myocardium
which arises in pathological changes in the heart proper or in the blood
vessels (heart defects, hypertension in the greater or lesser circulation). The
left or right ventricle, or the entire heart are overloaded due to various
causes.
The left ventricle is overloaded (1) in the presence of obstacles to blood ejection from the ventricle; this may be due to the narrowing of the aortic isthmus or orifice, or a sharp and persistent increase in the arterial pressure; (2) in diastolic overfilling of the left ventricle in patients with aortic incompetence or mitral insufficiency.
The right ventricle may be overloaded (1) in difficult blood outflow from this ventricle due to the narrowing of the pulmonary trunk orifice, or increased pressure in the lesser circulation; (2) in diastolic overfilling of the right ventricle associated with tricuspid or pulmonary valve incompetence.
Both ventricles are overloaded in combined or concurrent heart diseases, in some congenital heart diseases, in adhesive pericarditis, etc. Primary affections of the myocardium and its overloading sometimes contribute to the development of heart failure. Rheumatic myocarditis or rheumatic heart disease can, for example, become the cause of circulatory insufficiency in a rheumatic patient. Heart failure may be aggravated by various infections and intoxications, physical strain, pregnancy, injuries,
18*
Special Part
Chapter 6. Blood Circulatory System

 and surgical operations. The same factors can become a direct cause of heart failure in patients with heart diseases, cardiosclerosis, etc.
and surgical operations. The same factors can become a direct cause of heart failure in patients with heart diseases, cardiosclerosis, etc.
Abnormal activity of the heart can for a long time be compensated by its intensified work and also by some extracardiac factors that ensure adaptation of the entire circulatory system to the increased demands of the body: (1) the force of heart contractions is increased by the action of the vagus nerve; (2) heart rate increases because elevated pressure in the orifice of the vena cava accelerates the cardiac rhythm (Bainbridge reflex); (3) diastolic pressure decreases due to dilatation of arterioles and capillaries; this ensures a more effective systolic emptying of the heart; (4) utilization of oxygen by tissues increases.
But prolonged overloading on the myocardium decreases the cardiac output and increases the residual systolic blood volume. This causes ventricular overfilling during diastole since the ventricle has to accept also its normal portion of blood. Diastolic pressure in the ventricle increases, the ventricle becomes distended, and the so-called tonogenic dilatation of the myocardium occurs. This dilatation, and the accompanying distention of the muscle fibres, intensify, according to Starling, contractility of the myocardium, cause its hyperfunction, and lead to its hypertrophy. Compensatory hypertrophy of the myocardium increases the heart activity to maintain circulation for a long period of time.
Long-standing hypertrophy of the myocardium becomes the cause of its wear out, dystrophic, and sclerotic processes. These processes are also promoted by impaired blood supply to the myocardium, because hypertrophy of the heart is attended only by the increase in the weight of the myocardium, while the coronary vessels remain unchanged. The contractile power of the myocardium thus decreases and even its marked distention during diastole does not intensify contractility. Decrease in contractility and the tone of the myocardium are attended by a significant dilatation of the heart chambers, which is called myogenic dilatation (as distinct from the compensatory tonogenic dilatation). Myogenic dilatation can arise without preceding hypertrophy of the myocardium, in primary affections of the heart muscle (in myocarditis or myocardial infarction).
Tachycardia, which first develops as a compensatory mechanism to maintain the normal minute blood volume at decreased stroke volume, later becomes the cause of myocardial weakening, because diastole shortens in tachycardia and the time of restoration of the biochemical processes in the myocardium decreases.
Thus, dilatation and hypertrophy of the heart and tachycardia can only partially compensate for disorders in the cardiovascular system. As heart failure progresses, these compensatory mechanisms themselves become the source of harmful effects. Further decrease in myocardial contractility causes marked disorders in haemodynamics.
Haemodynamic changes. As myocardial contractility decreases and myogenic dilatation of the ventricle develops, diastolic ventricular pressure increases while systolic pressure falls because the ability of the ventricle to strain during diastole sharply diminishes. This decreases the cardiac output and the minute blood volume. The mass of the circulating blood usually increases proportionally to the degree of circulatory insufficiency. This is favoured by retention of sodium chloride and water in decreased renal filtration and increased reabsorption of sodium, and the increasing number of red blood cells (hypoxia is attended by intensified haemopoiesis to compensate for the developing insufficiency). The rate of blood flow decreases. Blood pressure changes as well: venous and capillary pressure increases in the greater circulation; arterial pressure remains normal or diastolic pressure slightly increases and the pulse pressure decreases.
Haemodynamic disorders are attended by abnormal gas exchange. Slowing of the blood flow rate increases oxygen absorption by the tissues; to 60—70 per cent of oxygen (instead of the normal 30 per cent) is consumed in capillaries. The arteriovenous difference in the oxygen content of the peripheral blood increases. Developing disorders in gas exchange associated with heart failure upset the carbohydrate metabolism. Lactic acid that is formed in skeletal muscles is decomposed only partly in insufficient oxygen supply to tissues; the blood content of lactic and pyruvic acids therefore increases. The increased content of lactic acid upsets acid-base equilibrium and decreases alkaline reserve. At the beginning of heart failure acidosis is compensated for because lactic acid displaces carbon dioxide which is removed from the body through the lungs. If pulmonary ventilation is upset and carbon dioxide is not expired in sufficient amount, decompensated acidosis develops.
Accumulation of underoxidized metabolites in the blood and intensified work of respiratory muscles intensify basal metabolism to complete a vicious circle: the body's demands in oxygen increase, whereas the circulatory system fails to meet them. Oxygen debt thus increases.
Haemodynamic and metabolic disorders account for various clinical signs of heart failure.
Clinical manifestations of heart failure. An early and specific symptom of circulatory insufficiency is dyspnoea. It is manifested by unreasonably accelerated and intensified respiration; dyspnoea develops at rest or during mild exercise. Dyspnoea arises in upset gas exchange and accumulation of underoxidized metabolites in the blood. It has already been said that lactic acid accumulated in the blood combines with bicarbonate alkalis to displace carbon dioxide which stimulates the respiratory centre to accelerate and deepen respiration. Especially pronounced disorders in gas exchange arise in blood congestion in the lesser circulation when the respiratory surface and the respiratory excursions of the lungs decrease.
Special Part

 Development of dyspnoea is also provoked by accumulation of liquid in the pleural and the abdominal cavities which interferes with the respiratory excursions of the lungs. During exercise dyspnoea markedly increases; it also becomes more pronounced after meals, and in the recumbent position of the patient. Grave dyspnoea sometimes occurs in attacks, which are called cardiac asthma.
Development of dyspnoea is also provoked by accumulation of liquid in the pleural and the abdominal cavities which interferes with the respiratory excursions of the lungs. During exercise dyspnoea markedly increases; it also becomes more pronounced after meals, and in the recumbent position of the patient. Grave dyspnoea sometimes occurs in attacks, which are called cardiac asthma.
Heart failure is often attended by cyanosis. The skin and the mucosa turn blue with increasing content of the reduced haemoglobin in capillary blood (over 50 g/1, or 5 g/100 ml), which, in contrast to oxyhaemoglobin, is dark. The dark blood is seen through the skin to colour it bluish; the colour is especially intense at sites where the skin is thinner (lips, cheeks, ear auricles). Cyanosis in circulatory insufficiency may be due to overfilling of vessels of the lesser circulation with blood and impaired arterialization of blood (the so-called central cyanosis). Peripheral cyanosis, however, occurs more frequently. It is connected with the slowing down of the blood flow and increased oxygen utilization by the tissues. Since the slowing down of the blood flow is more pronounced in parts of the body remote from the heart, the blue colour appears in the limbs, the ears, and the tip of the nose (acrocyanosis). Cyanosis is favoured by the widening of the venous network in the skin, increased volume of the circulating blood, and its increased haemoglobin content.
Oedema is an important sign of circulatory insufficiency. Its development in individuals with heart diseases depends on the following factors: (1) increased hydrostatic pressure in the capillaries and slowed blood flow which promote transudation of fluids into tissues; (2) abnormal hormonal regulation of the water-salt metabolism. Insufficient supply of arterial blood to the kidneys intensifies excretion of renin which increases secretion of adrenal cortex hormone, aldosterone. Aldosterone in turn increases the reabsorption of sodium in convoluted renal tubules to promote retention of fluid in the tissues. Furthermore, secretion of antidiuretic pituitary hormone increases to intensify re-absorption of water. These disorders in water-salt metabolism increase the volume of blood plasma, venous and capillary pressure, and intensify transudation of fluid in tissues; (3) during long-standing venous congestion in the great circulation, liver function decreases and the production of albumins becomes disordered to decrease oncotic pressure of blood plasma. Moreover, liver dysfunction inhibits the decomposition of the antidiuretic hormone and aldosterone in the liver.
Cardiac oedema can first be latent. Retention of fluid in the body (sometimes to 5 1 and more) does not immediately cause visible oedema but provokes a rapid gain in the patient's weight and his decreased urination. Oedema becomes visible in the first instance in the lower part of the body: in the lower limbs (if the patient sits or stands) and in the sacral region (if
the patient keeps bed). If circulatory insufficiency is progressive, oedema increases and dropsy of the body cavities develops. Fluid can be accumulated in the abdominal cavity (ascites), in the pleural cavity (hydrothorax), and in the pericardial cavity (hydropericardium). Ascites may be provoked by a prolonged venous congestion in the liver attended by its fibrosis and portal hypertension. Ascites then prevails over dropsy of other cavities.
Practically all other organs are changed in patients with heart failure. Changes in the lungs are connected with prolonged congestion of blood in the lesser circulation. Congested lungs become rigid to decrease respiratory excursions of the chest and limit mobility of the lower border of the lungs. The so-called congestive bronchitis thus develops. Patients develop cough; it may be dry or with expectoration of small amounts of mucous sputum; harsh respiration is heard over the lungs during auscultation; dry rales (intense in the postero-inferior parts of the chest) are heard; later they become moist. Long-standing venous congestion in the lesser circulation stimulates development of connective tissue in the lungs, which in turn impaires gas exchange. The overfilling of smaller vessels with blood may be accompanied by their rupture and the appearance of blood in the sputum. Insignificant haemorrhage, and also insinuation of erythrocytes through the blood vessels, promote deposition of the blood pigment in the lungs and development of brown induration; heart-failure cells can be detected in the sputum.
There are also some cardiovascular signs indicating inadequate contractility of the myocardium. Considerable myogenic dilatation of the heart chambers can cause relative insufficiency of atrioventricular valves. The heart borders broaden, the sounds of the heart (especially the first sound) weaken, tachycardia develops, and gallop rhythm sometimes occurs to suggest a grave affection of the myocardium and lowering of its tone. Organic murmurs usually weaken, because the blood-flow rate slows down; functional murmurs associated with relative atrioventricular incompetence may develop.
The liver quickly responds to venous congestion in the greater circulation. It becomes enlarged, Glisson's capsule distended, and the patient complains of the right hypochondrium pain. If congestion develops gradually, the patient feels heaviness in the epigastric and right hypochondrium. Prolonged venous congestion in the liver stimulates development of connective tissue (cardiac fibrosis of the liver) with subsequent hepatic dysfunction and portal hypertension.
The gastro-intestinal function is also impaired. Congestion in the greater circulation provokes congestive gastritis and intestinal dysfunction. Patients complain of poor appetite, nausea, and vomiting; they develop
Special Part
Chapter 6. Blood Circulatory System


 meteorism and constipation. Dyspeptic and metabolic disorders cause disturbances in nutrition; as circulatory insufficiency further progresses, grave asthenia (cardiac cachexia) develops.
meteorism and constipation. Dyspeptic and metabolic disorders cause disturbances in nutrition; as circulatory insufficiency further progresses, grave asthenia (cardiac cachexia) develops.
Venous congestion in the kidneys decreases the daily amount of the urine, and its specific gravity increases. A small amount of protein, red blood cells, and casts can be found in the urine.
Circulatory insufficiency soon becomes attended by dysfunction of the central nervous system. Rapid fatigue, decreased work capacity and mental power, high irritability, deranged sleep, and sometimes, depression are characteristic.
The clinical signs and changes in various organs of the body depend on the degree and duration of heart failure, and on the particular side of the heart that is affected (right or left).
Bearing these factors in mind, Strazhesko and Vasilenko provided their classification of circulatory insufficiency, which was adopted at the 12th All-Union Congress of Therapeutists in 1935. According to this classification, the following forms of circulatory insufficiency are distinguished.
1. Acute circulatory insufficiency. It can depend on acute heart failure
(either side) or failure of any of its chambers, (left or right ventricle, left
atrium), or else it may be caused by acute vascular insufficiency (collapse
or shock).
2. Chronic circulatory insufficiency. This can be divided into three
stages.
The first stage (initial) is latent circulatory insufficiency, which is only manifested during physical exercise, while at rest the haemodynamics and functions of the organs are normal; the work capacity is decreased.
The second stage is characterized by a pronounced prolonged circulatory insufficiency, haemodynamic disorders (congestion in the lesser or greater circulation) and dysfunction of organs at rest; the work capacity of patients is markedly decreased. Two periods are distinguished at this stage: (1) the initial period, with mild haemodynamic disorders; and (2) the final period characterized by grave haemodynamic disorders.
The third stage is the terminal or dystrophic stage of circulatory insufficiency. In addition to grave haemodynamic disorders, irreversible morphological changes develop in the organs along with persistent metabolic disorders and disability.
Clinical forms of heart failure. Acute heart failure may develop in grave disorders in the cardiac rhythm (paroxysmal tachycardia, ventricular fibrillation, myocardial infarction, acute myocarditis, and the like). Acute heart failure is attended by a marked drop in the minute volume and filling of the arterial system; clinically it is very much like circulatory insufficiency of the vascular genesis (it is sometimes termed as acute cardiac collapse). Clinically it is manifested by sudden and pronounced asthenia, sometimes
by syncopes due to brain ischaemia, pallidness and cyanosis of the skin, cold limbs, small or thready pulse, and decreased arterial pressure.
The cardiac aetiology of circulatory insufficiency is confirmed by changes in the heart proper (valve incompetence or arrhythmia, broadening of the heart borders, changes in the heart sounds and gallop rhythm). The attending venous congestion is manifested by dyspnoea, swelling of the neck veins, rales in the lungs, and enlargement of the liver. Acute heart failure may depend not on the weakening of the entire myocardium but on a pronounced decrease in contractile capacity of the myocardium of one of the heart chambers: left ventricle, left atrium, or right ventricle.
The syndrome of acute left-ventricular heart failure arises in patients in whom the left ventricle is mostly affected (essential hypertension, aortic incompetence, myocardial infarction). A typical symptom of acute left-ventricular heart failure is cardiac asthma (attacks of severe dyspnoea due to acute congestion in the lungs and upset gas exchange). Attacks can be provoked by physical exercise and nervous strain. Attacks usually occur during night sleep. This can be explained by an increased vagus tone during sleep, which causes narrowing of the coronary arteries and thus impairs nutrition of the myocardium. Moreover, blood supply to the respiratory centre decreases during sleep and its excitability diminishes. The lesser circulation becomes overfilled with blood because during a sharply decreased contractility of the left-ventricular myocardium, the right ventricle continues working intensely to pump the blood from the greater circulation to the lesser one.
During an attack of cardiac asthma, the patient develops asphyxia and marked weakness; cold sweat appears. He has to assume a forced position—sitting with his legs hanging down from the bed (or he stands up). The patient begins coughing and expectorates tenacious sputum. The skin becomes pallid and cyanotic. Moist and dry rales are heard over the lungs. The heart sounds are weakened at the apex and over the pulmonary artery intensified (the second sound). Tachycardia and small frequent pulse are characteristic. If congestion in the lesser circulation progresses, the blood plasma and blood corpuscles pass from the overfilled pulmonary capillaries to the alveoli and accumulate in the respiratory ducts; oedema of the lungs develops to intensify still more the feeling of suffocation and cough; respiration becomes rattling; ample foaming sputum with traces of blood (pink or red) is expectorated. Many moist rales of various calibres are heard over the lungs (over their entire surface). Auscultation of the heart often reveals gallop rhythm. Pulse is markedly accelerated and thready. Oedema of the lungs requires prompt and energetic measures to be taken to prevent possible death.
The syndrome of acute left-atrial failure develops in patients with mitral stenosis in markedly weakened contractility of the left atrium and
Special Part
Chapter 6. Blood Circulatory System